Sensory and Developmental Neurobiology
We study the development of the cochlea and its innervation because it is a model organ in which to elucidate fundamental principles of development and neuroscience. Our research aims at overcoming the obstacles that lay in the way towards therapeutic regeneration and restoration of lost hearing. Current projects address how inner and outer sensory hair cells form, how they assemble with specialized supporting cells into the cellular mosaic that is the organ of Corti, and how they get innervated in order to communicate with the brain.
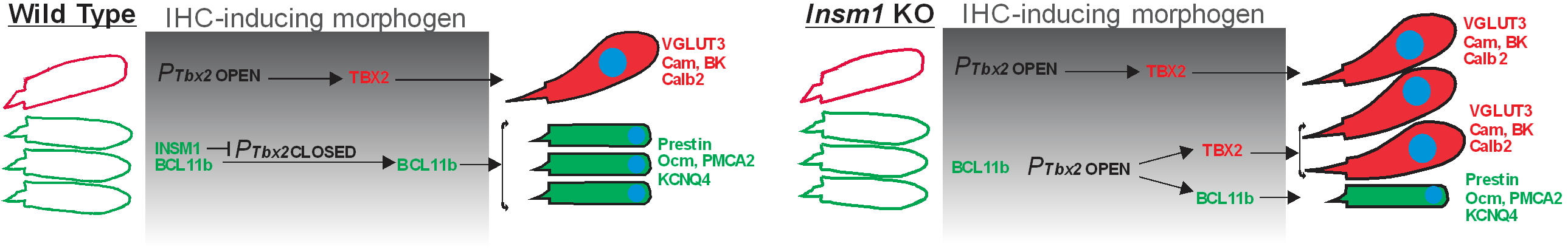
Development of inner and outer hair cells: The two mechanosensory cells in the cochlea, inner (IHC) and outer (OHC) hair cells, play complementary roles in hearing. IHCs, endowed with a prominent presynaptic machinery and densely innervated by afferent neurons, transmit auditory information to the brain. OHCs, which are electromotile, contract and expand when stimulated by sound and serve as amplifiers, permitting the detection of soft sounds and the discerning of sounds of similar frequencies. Cochlear hair cells are produced exclusively during an early developmental period, and hence their death throughout life, whether caused by age, noise or ototoxic drugs, result in irreversible deafness. Attempts at generating new hair cells from cochlear supporting cells are under way, but we need to know how to specifically generate IHCs and OHCs, each in their appropriate location. Our main contribution in the last few years has been towards elucidating how this cellular dichotomy arises during development as well as evolution. Prominent among these studies is our discovery of INSM1 in the consolidation of the fate of OHCs and of their transdifferentiation into IHCs in its absence (Wiwatpanit et al., 2018, Nature), followed by the discovery of TBX2 as a master regulator for IHC vs OHC differentiation (García-Añoveros et al., 2022, Nature). Essentially, we demonstrated that INSM1 acts in OHCs during a critical period to prevent them from responding to an IHC-inducing morphogen and activating TBX2, which will induce the formation of IHCs as opposed to OHCs (Fig 1). Having identified the major regulators, we are now in a position to elucidate how they execute their molecular functions and to use this information to drive the therapeutic generation of new hair cells into specifically forming IHCs or OHCs. Our expectation is to acquire a detailed understanding of the molecular mechanisms by which cochlear hair cells of either type are generated during normal development and can be generated with induced therapies.
Figure 1: Summary of the molecular mechanisms for the generation of IHCs vs OHCs.
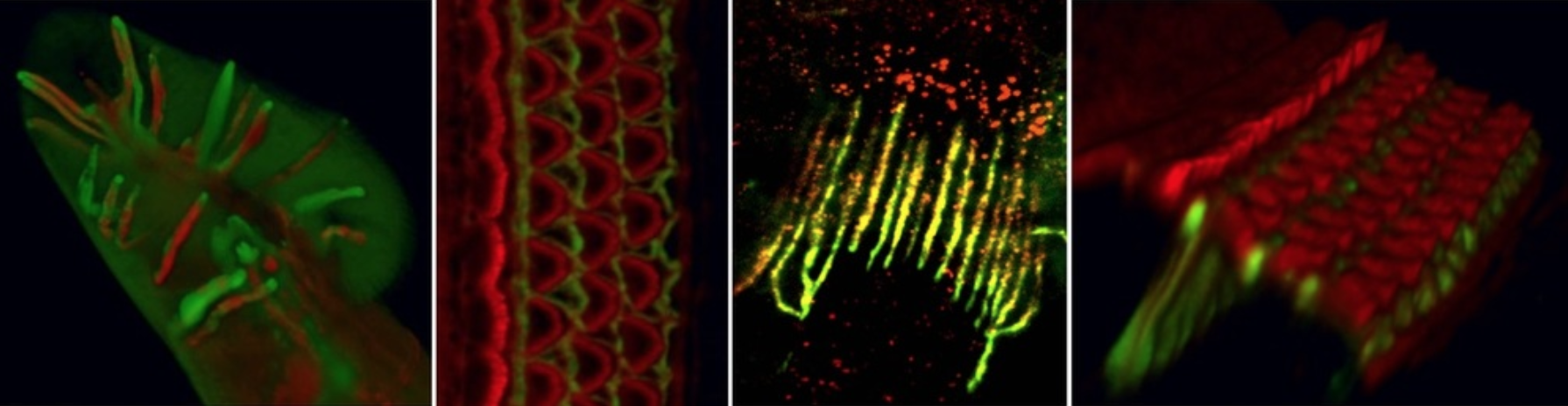
Intercellular arrangement of hair and specialized supporting cells. To perform their role in hearing, hair cells must be placed in very precise ways. IHCs form a first row, so as to receive most of the innervation through which they communicate sound information to the brain. OHCs appear more laterally, interlaced with special supporting cells in an arrangement in which their electromotility will result in sound amplification and tuning. This micrometrically precise intercellular distribution is critical for their contribution to hearing. We are exploring how the specialized supporting cells for IHCs and OHC are generated and assemble with them into the near crystalline mosaic that is the organ of Corti. This basic information will be critical for any regenerative attempt at restoring hearing; it will also reveal fundamental mechanisms of how multiple cell types assemble into a coherent functional structure, i.e., an organ.
Figure 2: Mosaic intercellular arrangement of the organ of Corti and indication of the specialized supporting cell types paired with, and separating, IHCs and OHCs.
Assembly of cochlear neural circuits. The cochlea communicates with the brain via an iterative but relatively simple circuit composed of a distinct pair of afferent plus efferent neurons for IHCs, and another for OHCs. Elucidating how this innervation develops is crucial for reconnecting hair cells generated via regenerative therapies. We also believe that the moderate complexity of cochlear innervation makes it an ideal system in which to study neuronal circuit assembly. Taking advantage of our ability to interconvert IHCs into OHCs and vice versa, we begun to discover general steps for the formation of this circuit (Webber et al., 2021, Science Advances; Fig 3). With this approach we expect to elucidate first the general principles, and then the molecular mechanisms, guiding the development of cochlear innervation, essential for hearing and paradigmatic for neuroscience.
Figure 3: Innervation of the cochlea and the sequence of signals (attractive in green and repulsive in red) by which it is achieved.
Cochlear type II afferents. While cochlear type I afferents communicate auditory information from IHCs to brain, the much less abundant type II afferents contact OHCs, but the type of information they conveyed is open to debate. With a simple genetic model, we suggested that they may convey auditory nociception, the detection of damage caused by intense sounds (Flores et al., 2015, Current Biology). With out ability to selectively eliminate the function and innervation of IHCs vs OHCs, we are further testing this and alternative hypotheses. These studies promise to resolve a long-held question in the field, the physiological function of the afferents innervating the OHCs, and may help us address pain hyperacusis, the pathological perception of sounds as painful.
We study the development of the cochlea and its innervation because it is a model organ in which to elucidate fundamental principles of development and neuroscience. Our research aims at overcoming the obstacles that lay in the way towards therapeutic regeneration and restoration of lost hearing. Current projects address how inner and outer sensory hair cells form, how they assemble with specialized supporting cells into the cellular mosaic that is the organ of Corti, and how they get innervated in order to communicate with the brain.
Development of inner and outer hair cells: The two mechanosensory cells in the cochlea, inner (IHC) and outer (OHC) hair cells, play complementary roles in hearing. IHCs, endowed with a prominent presynaptic machinery and densely innervated by afferent neurons, transmit auditory information to the brain. OHCs, which are electromotile, contract and expand when stimulated by sound and serve as amplifiers, permitting the detection of soft sounds and the discerning of sounds of similar frequencies. Cochlear hair cells are produced exclusively during an early developmental period, and hence their death throughout life, whether caused by age, noise or ototoxic drugs, result in irreversible deafness. Attempts at generating new hair cells from cochlear supporting cells are under way, but we need to know how to specifically generate IHCs and OHCs, each in their appropriate location. Our main contribution in the last few years has been towards elucidating how this cellular dichotomy arises during development as well as evolution. Prominent among these studies is our discovery of INSM1 in the consolidation of the fate of OHCs and of their transdifferentiation into IHCs in its absence (Wiwatpanit et al., 2018, Nature), followed by the discovery of TBX2 as a master regulator for IHC vs OHC differentiation (García-Añoveros et al., 2022, Nature). Essentially, we demonstrated that INSM1 acts in OHCs during a critical period to prevent them from responding to an IHC-inducing morphogen and activating TBX2, which will induce the formation of IHCs as opposed to OHCs (Fig 1). Having identified the major regulators, we are now in a position to elucidate how they execute their molecular functions and to use this information to drive the therapeutic generation of new hair cells into specifically forming IHCs or OHCs. Our expectation is to acquire a detailed understanding of the molecular mechanisms by which cochlear hair cells of either type are generated during normal development and can be generated with induced therapies.
Figure 1: Summary of the molecular mechanisms for the generation of IHCs vs OHCs.
Intercellular arrangement of hair and specialized supporting cells. To perform their role in hearing, hair cells must be placed in very precise ways. IHCs form a first row, so as to receive most of the innervation through which they communicate sound information to the brain. OHCs appear more laterally, interlaced with special supporting cells in an arrangement in which their electromotility will result in sound amplification and tuning. This micrometrically precise intercellular distribution is critical for their contribution to hearing. We are exploring how the specialized supporting cells for IHCs and OHC are generated and assemble with them into the near crystalline mosaic that is the organ of Corti. This basic information will be critical for any regenerative attempt at restoring hearing; it will also reveal fundamental mechanisms of how multiple cell types assemble into a coherent functional structure, i.e., an organ.
Figure 2: Mosaic intercellular arrangement of the organ of Corti and indication of the specialized supporting cell types paired with, and separating, IHCs and OHCs.
Assembly of cochlear neural circuits. The cochlea communicates with the brain via an iterative but relatively simple circuit composed of a distinct pair of afferent plus efferent neurons for IHCs, and another for OHCs. Elucidating how this innervation develops is crucial for reconnecting hair cells generated via regenerative therapies. We also believe that the moderate complexity of cochlear innervation makes it an ideal system in which to study neuronal circuit assembly. Taking advantage of our ability to interconvert IHCs into OHCs and vice versa, we begun to discover general steps for the formation of this circuit (Webber et al., 2021, Science Advances; Fig 3). With this approach we expect to elucidate first the general principles, and then the molecular mechanisms, guiding the development of cochlear innervation, essential for hearing and paradigmatic for neuroscience.
Figure 3: Innervation of the cochlea and the sequence of signals (attractive in green and repulsive in red) by which it is achieved.
Cochlear type II afferents. While cochlear type I afferents communicate auditory information from IHCs to brain, the much less abundant type II afferents contact OHCs, but the type of information they conveyed is open to debate. With a simple genetic model, we suggested that they may convey auditory nociception, the detection of damage caused by intense sounds (Flores et al., 2015, Current Biology). With out ability to selectively eliminate the function and innervation of IHCs vs OHCs, we are further testing this and alternative hypotheses. These studies promise to resolve a long-held question in the field, the physiological function of the afferents innervating the OHCs, and may help us address pain hyperacusis, the pathological perception of sounds as painful.